The New Axis of Disease: How Oral Dysbiosis Initiates Systemic Illness and What You Can Do to Save Lives, Every Day!
Introduction
The oral microbiome has been recently classified as a “Gateway” microbiome, giving it immense importance in total body health.(1) The placental microbiome is another gateway microbiome, that sets the immune system of the developing child, and is also extremely important. Not surprisingly it is most closely related in microbial content to the oral microbiome!(2) This scenario makes the oral microbiome most crucial for health and the most important in treatment priority. But what creates a dysbiotic oral microbiome?
Antimicrobials, both dietary and therapeutic can eliminate many protective commensals. (3-4) A sugar heavy diet supporting pathogen growth, very poor oral hygiene practices, severe dental crowding, and very importantly, mouth breathing can all negatively affect the oral microbiome. (5-6) Nutritional counseling, prebiotic and probiotic supplementation along with correction of the mouth breathing are all necessary for long term oral and systemic health. (7-8) An oral probiotic with decades of positive research is available that uses three probiotic strains to significantly improve the oral microbiome (9-11), literally changing hundreds of the other oral microbial strains! The new axis of disease consists of the following; oral dysbiosis leads to placental dysbiosis, oral dysbiosis also leads to gut dysbiosis (leaky gut) and leaky blood brain barrier. (12-14) All of this has been well accepted by the medical community because of definitive recently published research.
This recently published research has revealed the precise mechanisms used by key pathogens to suppress the host’s immune system creating a slew of systemic illnesses. (15-17) Indeed, key oral pathogens are now proven to be the determinants of the new axis of disease. With an oral dysbiosis comes an eventual gut dysbiosis leading to “leaky” gut and faulty Blood Brain Barrier. The connection has been well defined between these key pathogens and metabolic syndrome, premature birth, miscarriages, atherosclerosis, inflammatory Alzheimer’s and cancer.(18) Fortunately, we have an armamentarium of prebiotics and probiotics that can help oral healthcare professionals restore an oral eubiosis! In addition, early diagnosis and correction of mouth breathing can also benefit the oral microbiome. (19-20) Impressive new research into the many benefits of probiotics will be presented. And the surprising effect of probiotics and the microbiome affecting cancer is truly encouraging and not to be overlooked.(21)
This article will provide all the research-based information necessary to establish proper oral microbiome support in preventing dental caries, periodontal disease, metabolic syndrome, atherosclerosis, inflammatory Alzheimer’s and other associated illnesses. The latest in research proving the necessity of newer and more effective prevention protocols will be presented. In short, this is how we try to save lives, every day.
Background
How does dysbiosis occur? Unfortunately, western diet and industrialization has further influenced human health by corrupting our diet. (22) The first major change for homo sapiens happened as the hunter gatherers became horticulturists, and later even more with the Neolithic period and the advent of agriculture (grain diet).(23) Now we have the introduction of fast food and processed food, coinciding with the over-use of antibiotics, further affecting the health of humans and domestic animals.(24) Even certain anti-microbial mouth-rinses have sadly reduced our oral commensal bacteria.(25) We are truly missing many of our “old friends” when it comes to the oral and gut microbiomes. A recent study of Hadza infants demonstrated that over 23% of their gut microbiome contained bacteria that were “novel” to western nation infants.(26) Previously published research revealed that children with the diagnosis of Autism Spectrum Disease have a microbiome deficient in protective bacteria but also had pathogenic bacteria capable of affecting neurotransmitter levels theoretically necessary for “normal” neural development. (27-30)
Key pathogens
Scardovia wiggsiae is a Bacillus bacterium found extensively associated with
Severe-Early Childhood Caries. Scardovia wiggsiae and Slackia exigua have
both been reported to be involved in the early caries development.(31) Candida albicans, a fungal organism, helps with the biofilm production by increasing the extracellular polysaccharide matrix which protects Streptococcus mutans from anti-microbials and commensals such as Streptococcus oralis. (32) Lactobacilli inhibit the colonization of Candida albicans, hence decreasing the polysaccharide matrix, exposing the Streptococcus mutans to the bactericins or hydrogen peroxide of its natural competitors, other Streptococcus species. Streptococcus oralis produces hydrogen peroxide that inhibits the anaerobic Streptococcus mutans growth. (33) Indeed, Probiora probiotic, a commercially available probiotic product, contains Streptococcus oralis, uberis and rattus, and claims to inhibit several key dental pathogens. Probiotics have been reported to be an important adjunct in preventive dental care. (34-36)
Another key pathogen is Porphyromonas gingivalis. P gingivalis is an anaerobic bacterium belonging to the phylum Bacteroidetes and is a nonmotile, Gram-negative, rod-shaped, and pathogenic. Being anaerobic it is inhibited by hydrogen peroxide producing Streptococcus species such as, S. oralis and S.uberis. Porphyromonas gingivalis has been reported to be a causative agent of periodontal disease, pre-term birth, metabolic syndrome, arteriosclerosis, and inflammatory Alzheimer’s. Because P gingivalis can be considered the foremost or “keystone” initiator of periodontal disease, it is reasonable to describe it as the causal agent of Neural Arterial Gingival Simplex, a single disease with all of its associated comorbidities. (37)
P. gingivalis has also been called a “guerilla” for its notable tactics of slowly subverting the host’s defensive mechanisms. It does this in several ways:
• The host’s immunity is bypassed by the ability of P. gingivalis fimbriae to attach to hosts’ cells, like gingival epithelial cells or endothelial cells, and then invade the cell itself. Intra-cellular invasion prevents macrophages from defending the host tissue. The invaded host cell often becomes senescent, and the endopathobiont P. gingivalis remains relatively inactive until the host cell’s apoptosis.(17)
• P. gingivalis has the ability to shift genomes in different strains to specifically target different host cells, making it particularly virulent and destructive.(15)
• The epigenetic influence of P. gingivalis allows it to open the tight junctions between cells and to modulate the immune response. In addition, P. gingivalis intracellular invasion of macrophages increases the macrophages’ nitric oxide production, increasing capillary growth.(16)
In summary, P. gingivalis undermines a massive host immune response but does not normally overwhelm the host because that would effectively limit the spread of the pathogen.(17) A dead host does not help a pathogen flourish.
With the new concept of P. gingivalis infection creating a systemic single disease with multiple symptoms, it is easy to understand the processes involved. (37) The oral component houses the initial infection where the immune system is alerted and subverted, creating an inflammatory environment resulting with periodontal disease. Circulating leukocytes may carry P. gingivalis and associated lipopolysaccharides, which affect the endothelial cells of arteries, and infect the arterial walls and neural tissues, compromising the blood brain barrier. (38) This eventually diminishes the cognitive ability of the host, resulting in reduced oral hygiene and further spreading of the P. gingivalis pathogen amongst all the host’s contacts.
Prebiotics are substances that promote probiotics and possibly also inhibit pathogens. The most commonly utilized prebiotics are polyols, such as, xylitol and erythritol. Numerous studies have demonstrated the effectiveness of polyols in inhibiting cariogenic and periodontal pathogens, (39-42) Fortunately, xylitol is available in many forms making incorporation into preventatives relatively achievable. Xylitol toothpaste, mouth rinse, lollipops, candy, nasal spray, and gum is commercially available and very well accepted by patients. Studies have reported that xylitol can also prevent the transmission of cariogenic bacteria from mother to child. (43) The preventive effect, reduction in the DMFT was significantly better than the “positive” control of fluoride varnish.(44) Interestingly, xylitol is also anti-inflammatory and has proven cancer inhibitory effects, increasing the apoptosis of cancer cells and preventing angiogenesis in the tumor.(45-48) Xylitol in a nasal spray has also been documented to reduce respiratory viral load with several key viral pathogens.(49) This is in addition to the benefits of improving nasal respiration quality.(50)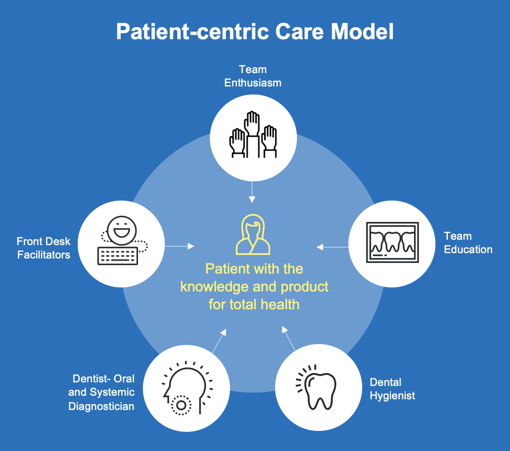
Successful implementation of a modern preventive program in dental practice must include consideration of oral and systemic health. The most important step, the very first step in implementing a modern preventive program is to totally involve the entire office staff. The whole team should be educated as to the value of “saving lives every day”. Indeed, most team members will be very enthusiastic about their new roles as total body health professionals. This requires an investment into education, as they not only need to know what to do, but why and for whom.
BRING YOUR TEAM TO AAOSH MEETINGS!
During the examinations and especially during the dental prophylaxis, the conversations should be devoted to how oral health determines gut health, and how gut health effects total systemic health. Many patients are surprised to hear the published research and are very grateful that their oral professionals are so current in their health knowledge. The frequent comment is “why am I hearing this for the first time, and why didn’t my previous dental professional tell me?” Dental hygienists have a pivotal role in this crucial period of time, reinforcing the importance of oral and systemic health. Team members can often significantly help by providing brochures, and in the case of my office, we use tear sheets with key points checked and comments circled. Having appropriate brochures and literature is very helpful for take home notes for the patients. Also, products and brochures should be prominently displayed. As the patients are walked to the reception area, key points should be reiterated and the suggested product available at the desk. Patients often will not purchase needed preventative product if it is inconvenient, so minimize the patient’s effort by always having preventative prebiotics and probiotics readily available.
The benefits are obvious to the patients and team members as oral pathology is obviously reduced in the compliant patients. The cavity rate and gingival health is always improved if the patient follows the suggested protocols. If they do come back and there is oral disease, they will most likely confess that they had inadvertently slacked off on the recommended product/protocol. In many offices, the profits from the sale of product goes into a general office fund that supports office events and education. That is a win/win for all, patients, doctors, and office team.
Conclusion
The best defense against oral and systemic disease is a healthy oral microbiome. Essential for the health of the oral microbiome, the Oral Gateway microbiome, is the significant presence of beneficial bacteria that inhibit the key pathogens. The only clinically available preventative that re-establishes the healthy oral microbiome would be an effective oral probiotic product. ProBiora is a commercially available product that contains three very important probiotic bacteria that inhibits the key pathogenic bacteria and maintains the healthy oral microbiome for total oral and systemic health. 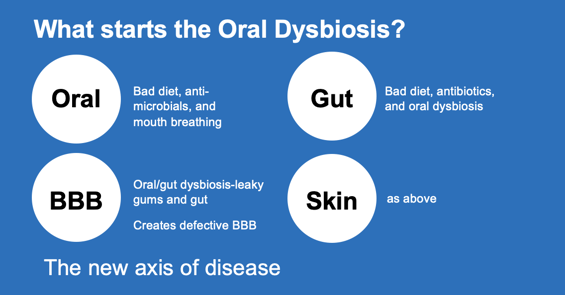

Resources
View the sample ProBiora script pad tear sheet that is available for hygienists to write out to their patients by clicking here.

Scan above or visit www.ProBioraHealth.com/dental to learn more and order ProBiora probiotics for the mouth today.
References
1. Nobel Assembly June 1-2nd., 2017-The microbiome and its programming of body systems. No 64.
2. Aagaard, Kjersti et al. “The placenta harbors a unique microbiome.” Science translational medicine vol. 6,237 (2014): 237ra65. doi:10.1126/scitranslmed.3008599
3. Marques TM, Cryan JF, Shanahan F, Fitzgerald GF, Ross RP, et al. Gut microbiota modulation and implications for host health: dietary strategies to influence the gut-brain axis (2014) Innov Food Sci Emerg Technol 22: 239-47. https://doi.org/10.1016/j.ifset.2013.10.016
4. Walsh CJ, Guinane CM, OToole PW and Cotter PD. Beneficial modulation of the gut microbiota (2014) FEBS Lett 588: 4120-4130. https://doi.org/10.1016/j.febslet.2014.03.035
5. Okada H, Kuhn C, Feillet H and Bach JF. The hygiene hypothesis for autoimmune and allergic diseases: an update (2010) Clin Exp Immunol 160: 1-9. https://doi.org/10.1111/j.1365-2249.2010.04139.x
6. Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists (2017) Cancer Res 77: 1783-1812. https://doi.org/10.1158/0008-5472.CAN-16-2929
7. Swartwout B and Luo XM. Implications of Probiotics on the Maternal-Neonatal Interface: Gut Microbiota, Immunomodulation, and Autoimmunity (2018) Front Immunol 9: 2840. https://doi.org/10.3389/fimmu.2018.02840
8. Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF, et al. Probiotics, prebiotics and amelioration of diseases (2019) J biomed sci 26: 3. https://doi.org/10.1186/s12929-018-0493-6
9. Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, et al. Influence of diet on the gut microbiome and implications for human health (2017) J Transl Med 15: 73 https://doi.org/10.1186/s12967-017-1175-y
10. Hillman JD, Socransky SS (1989) The theory and application of bacterial interference to oral diseases. In New Biotechnology in Oral Research. ed. Myers HM (Basel: S Karger) 1-17.
11. Zahradnik RT, Magnusson I, Walker C, McDonell E, Hillman CH (2009) Preliminary assessment of safety and effectiveness in humans of ProBiora3, a probiotic mouthwash. J Applied Micro 107(2): 682-690.
12. Hajishengallis G, Darveau RP and Curtis MA. The keystone-pathogen hypothesis (2012) Nat Rev Microbiol 10: 717-725. https://dx.doi.org/10.1038%2Fnrmicro2873
13. Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? (2009) Microbes infect 11: 637-645. https://doi.org/10.1016/j.micinf.2009.03.009
14. Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, et al. Porphyromonas gingivalis in Alzheimers disease brains: Evidence for disease causation and treatment with small-molecule inhibitors (2019) Sci adv 5: eaau3333. https://doi.org/10.1126/sciadv.aau3333
15. Tribble GD, Kerr J E, and Wang BY. Genetic diversity in the oral pathogen Porphyromonas gingivalis: molecular mechanisms and biological consequences (2013) Future microbial 8: 607-620. https://doi.org/10.2217/fmb.13.30
16. Guo W, Wang P, Liu ZH, and Ye P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate (2018) Int J oral sci 10: e8. https://doi.org/10.1038/ijos.2017.51
17. Hajishengallis G and Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis (2014) Eur J Immunol, 44: 328-338. https://doi.org/10.1002/eji.201344202
18. Jin, L. J., Lamster, I. B., Greenspan, J. S., Pitts, N. B., Scully, C., & Warnakulasuriya, S. (2016). Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral diseases, 22(7), 609–619. https://doi.org/10.1111/odi.12428
19. Zamarron, C., García Paz, V., & Riveiro, A. (2008). Obstructive sleep apnea syndrome is a systemic disease. Current evidence. European journal of internal medicine, 19(6), 390–398. https://doi.org/10.1016/j.ejim.2007.12.006
20. Fan, C., Guo, L., Gu, H., Huo, Y., & Lin, H. (2020). Alterations in Oral-Nasal-Pharyngeal Microbiota and Salivary Proteins in Mouth-Breathing Children. Frontiers in microbiology, 11, 575550. https://doi.org/10.3389/fmicb.2020.575550
21. Gopalakrishnan, V., Helmink, B. A., Spencer, C. N., Reuben, A., & Wargo, J. A. (2018). The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer cell, 33(4), 570–580. https://doi.org/10.1016/j.ccell.2018.03.015
22. Malesza, I. J., Malesza, M., Walkowiak, J., Mussin, N., Walkowiak, D., Aringazina, R., Bartkowiak-Wieczorek, J., & Mądry, E. (2021). High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells, 10(11), 3164. https://doi.org/10.3390/cells10113164
23. Stefano, G. B., Fine, R., & Kream, R. M. (2018). Microbiome and Health: Ramifications of Intelligent Deception. Medical science monitor : international medical journal of experimental and clinical research, 24, 2060–2062. https://doi.org/10.12659/msm.910248
24. Grech, A., Collins, C. E., Holmes, A., Lal, R., Duncanson, K., Taylor, R., & Gordon, A. (2021). Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut microbes, 13(1), 1–30. https://doi.org/10.1080/19490976.2021.1897210
25. Kapil, V., Haydar, S. M., Pearl, V., Lundberg, J. O., Weitzberg, E., & Ahluwalia, A. (2013). Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free radical biology & medicine, 55, 93–100. https://doi.org/10.1016/j.freeradbiomed.2012.11.013
26. Olm, M. R., Dahan, D., Carter, M. M., Merrill, B. D., Yu, F. B., Jain, S., Meng, X., Tripathi, S., Wastyk, H., Neff, N., Holmes, S., Sonnenburg, E. D., Jha, A. R., & Sonnenburg, J. L. (2022). Robust variation in infant gut microbiome assembly across a spectrum of lifestyles. Science (New York, N.Y.), 376(6598), 1220–1223. https://doi.org/10.1126/science.abj2972
27. MacFabe DF, Cain NE, Boon F, Ossenkopp KP and Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder (2011) Behav Brain Res 217: 47-54. https://doi.org/10.1016/j.bbr.2010.10.005
28. Rose S, Bennuri SC, Davis JE, Wynne R, Slattery JC, et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism (2018) Translational Psychiatry 8: 42. https://doi.org/10.1038/s41398-017-0089-z
29. Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites (2009) Proceedings of the National Academy of Sciences of the United States of America, USA 106: 3698-3703. https://doi.org/10.1073/pnas.0812874106
30. Argou-Cardozo I and Zeidán-Chuliá F. Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels (2018) Med Sci (Basel) 6: 29. https://doi.org/10.3390/medsci6020029
31. Fakhruddin, K. S., Ngo, H. C., & Samaranayake, L. P. (2019). Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral diseases, 25(4), 982–995. https://doi.org/10.1111/odi.12932
32. Xiao, J., Huang, X., Alkhers, N., Alzamil, H., Alzoubi, S., Wu, T. T., Castillo, D. A., Campbell, F., Davis, J., Herzog, K., Billings, R., Kopycka-Kedzierawski, D. T., Hajishengallis, E., & Koo, H. (2018). Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries research, 52(1-2), 102–112. https://doi.org/10.1159/000481833
33. Hillman, J. D., Socransky, S. S., & Shivers, M. (1985). The relationships between streptococcal species and periodontopathic bacteria in human dental plaque. Archives of oral biology, 30(11-12), 791–795. https://doi.org/10.1016/0003-9969(85)90133-5
34. Cannon M (2011) A Review of Probiotic Therapy in Preventive Dental Practice. Probiotics Antimicrob Proteins 3(2): 63-67.
35. Cannon M, Trent B, Vorachek A, Kramer S, Esterly R (2013) Effectiveness of CRT at measuring the salivary level of bacteria in caries prone children with probiotic therapy. J Clin Pediatr Dent 38(1): 55-60.
36. Cannon M (2011) Clinical Application of Probiotic Therapy. Inside Dentistry 7(6): 112-113.
37. Cannon ML and Peldyak JN. The prevention and treatment of neural arterial gingival simplex (2019) Dental Res Manag 3: 32-37
38. Xu, T., Dong, Q., Luo, Y., Liu, Y., Gao, L., Pan, Y., & Zhang, D. (2021). Porphyromonas gingivalis infection promotes mitochondrial dysfunction through Drp1-dependent mitochondrial fission in endothelial cells. International journal of oral science, 13(1), 28. https://doi.org/10.1038/s41368-021-00134-4
39. Janakiram C, Deepan Kumar CV and Joseph J. Xylitol in preventing dental caries: a systematic review and meta-analyses (2017) J Nat Sci Biol Med 8: 16-21. https://doi.org/10.4103/0976-9668.198344
40. Badet C, Furiga A and Thébaud N.Effect of xylitol on an in vitro model of oral biofilm (2008) Oral Health Prev Dent 6: 337-341.
41. Janus MM, Volgenant CMC, Brandt BW, Buijs MJ, Keijser BJF, et al. Effect of erythritol on microbial ecology of in vitro gingivitis biofilms (2017)J Oral Microbiol 9: 1. https://doi.org/10.1080/20002297.2017.1337477
42. Söderling E, Hietala and Lenkkeri AM. Xylitol and erythritol decrease adherence of polysaccharide-producing oral streptococci (2010) Curr Microbiol 60: 22-29. https://doi.org/10.1007/s00284-009-9496-6
43. Söderling E, Isokangas P, Pienihäkkinen K and Tenovuo J. Influence of maternal xylitol consumption on acquisition of mutans streptococci by infants (2000) J Dent Res 79: 882-887. https://doi.org/10.1177/00220345000790031601
44. Isokangas, P., Söderling, E., Pienihäkkinen, K., & Alanen, P. (2000). Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. Journal of dental research, 79(11), 1885–1889. https://doi.org/10.1177/00220345000790111201Xylitol cancer
45. Tomonobu, N., Komalasari, N., Sumardika, I. W., Jiang, F., Chen, Y., Yamamoto, K. I., Kinoshita, R., Murata, H., Inoue, Y., & Sakaguchi, M. (2020). Xylitol acts as an anticancer monosaccharide to induce selective cancer death via regulation of the glutathione level. Chemico-biological interactions, 324, 109085. https://doi.org/10.1016/j.cbi.2020.109085
46. Park, E., Park, M. H., Na, H. S., & Chung, J. (2015). Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnology letters, 37(5), 983–990. https://doi.org/10.1007/s10529-014-1757-1
47. Sahasakul, Y., Angkhasirisap, W., Lam-Ubol, A., Aursalung, A., Sano, D., Takada, K., & Trachootham, D. (2022). Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer. Nutrients, 14(10), 2023. https://doi.org/10.3390/nu14102023
48. Trachootham, D., Chingsuwanrote, P., Yoosadiang, P., Mekkriangkrai, D., Ratchawong, T., Buraphacheep, N., Kijanukul, S., Saekhow, S., Pongpitchayadej, O., Vongvachvasin, K., Sittikornpaiboon, P., & Tuntipopipat, S. (2017). Partial Substitution of Glucose with Xylitol Suppressed the Glycolysis and Selectively Inhibited the Proliferation of Oral Cancer Cells. Nutrition and cancer, 69(6), 862–872. https://doi.org/10.1080/01635581.2017.1339097
49. Mark L Cannon, Jonna B. Westover, Reiner Bleher, Marcos A. Sanchez-Gonzalez, Gustavo Ferrer In Vitro Analysis of the Anti-viral Potential of nasal spray constituents against SARS-CoV-2 bioRxiv 2020.12.02.408575; doi: https://doi.org/10.1101/2020.12.02.408575
50. Rabago, D., Kille, T., Mundt, M., & Obasi, C. (2020). Results of a RCT assessing saline and xylitol nasal irrigation for CRS and fatigue in Gulf War illness. Laryngoscope investigative otolaryngology, 5(4), 613–620. https://doi.org/10.1002/lio2.425
Additional Information
Mendrick DL, Diehl AM, Topor LS, Dietert RR, Will Y, et al. Metabolic Syndrome and Associated Diseases: From the Bench to the Clinic (2017) Toxicol Sci 162: 36-42.
Kanherkar RR, Bhatia-Dey N and Csoka AB. Epigenetics across the human lifespan (2014) Front Cell Dev Biol 2: 49. https://dx.doi.org/10.3389%2Ffcell.2014.00049
Li Y and Tollefsbol TO. Age-related epigenetic drift and phenotypic plasticity loss: implications in prevention of age-related human diseases (2018) Epigenomics 8:1637-1651. https://doi.org/10.2217/epi-2016-0078
Kim J and Amar S. Periodontal disease and systemic conditions: a bidirectional relationship (2006) Odontology 94: 10-21. https://dx.doi.org/10.1007%2Fs10266-006-0060-6
Kim HJ, Cha GS, Kim HJ, Kwon EY, LeeJY, et al. Porphyromonas gingivalis accelerates atherosclerosis through oxidation of high-density lipoprotein (2018) J periodontal implant sci 48: 60-68. https://doi.org/10.5051/jpis.2018.48.1.60
Bale BF, Doneen AD and Vigerust DJ. High-risk periodontal pathogens contribute to the pathogenesis of atherosclerosis (2016) Postgrad Med J 93: 215-220. https://doi.org/10.1136/postgradmedj-2016-134279
Hussain M, Stover CM and Dupont AP. Gingivalis in Periodontal Disease and Atherosclerosis - Scenes of Action for Antimicrobial Peptides and Complement (2015) Front Immunol 6: 45. https://doi.org/10.3389/fimmu.2015.00045
Moreno S and Contreras A. Functional differences of Porphyromonas gingivalis Fimbriae in determining periodontal disease pathogenesis: a literature review (2013) Colombia medica 44: 48-56.
Lamell CW, Griffen AL, McClellan DL and Leys EJ. Acquisition and colonization stability of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in children (2000) J Clin Microbi0l 38: 1196-1199.
Liu Y, Zhang Y, Wang, L, Guo Y, and Xiao S. Prevalence of Porphyromonas gingivalis four rag locus genotypes in patients of orthodontic gingivitis and periodontitis (2013) PloS one 8: e61028. https://dx.doi.org/10.1371%2Fjournal.pone.0061028
Toledo BE, Barroso EM, Martins AT and Zuza, EP. Prevalence of Periodontal Bone Loss in Brazilian Adolescents through Interproximal Radiography (2012) Int J Dent 2012: 357056. https://doi.org/10.1155/2012/357056
Rao D, Sood D, Pathak P, and Dongre SD. A cause of Sudden Cardiac Deaths on Autopsy Findings; a Four-Year Report (2014) Emergency 2: 12-17.
Hong YM. Atherosclerotic cardiovascular disease beginning in childhood (2010) Korean Circ J 40: 1-9. https://doi.org/10.4070/kcj.2010.40.1.1
Berenson GS, Srinivasan SR, Bao W, Newman WP and Tracy RE. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults (1998) Engl J Med 338: 1650-1656 https://doi.org/10.1056/NEJM199806043382302
Baker-Nigh A, Vahedi S, Davis EG, Weintraub S, Bigio EH, et al. Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimers disease (2015) Brain 138: 1722-1737. https://doi.org/10.1093/brain/awv024
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation (2015) Nat Rev Immunol 15: 30-44. https://doi.org/10.1038/nri3785
Barengolts E. Gut Microbiota, Prebiotics, Probiotics, And Synbiotics In Management Of Obesity And Prediabetes: Review Of Randomized Controlled Trials (2016) Endoc Prac 22: 1224-1234. https://doi.org/10.4158/EP151157.RA
Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study (2017) Microbiome 5: 10. https://doi.org/10.1186/s40168-016-0225-7
Mach N and Fuster-Botella D. Endurance exercise and gut microbiota: A review (2016) J Sport Health Sci 6: 179-197. https://doi.org/10.1016/j.jshs.2016.05.001
Qi Y and Wade PA. Crosstalk between the microbiome and epigenome: messages from bugs (2017) J Biochem1 63: 105-112. https://doi.org/10.1093/jb/mvx080
Kelly JR, Minuto C, Cryan JF, Clarke G and Dinan, TG. Cross Talk: The Microbiota and Neurodevelopmental Disorders (2017) Front Neurosci 11: 490. https://doi.org/10.3389/fnins.2017.00490
Lach G, Schellekens H, Dinan TG and Cryan JF. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides (2017) Neurotherapeutics 15: 36-59. https://doi.org/10.1007/s13311-017-0585-0
Strandwitz P, Kim KH, Terekhova D, Liu JK, Anukriti Sharmaet, et al. GABA-modulating bacteria of the human gut microbiota (2019) Nature Microbiol 4: 396-403. https://www.nature.com/articles/s41564-018-0307-3
Föcking M, Doyle B, Munawar N, Dillon ET, Cotter D, et al. Epigenetic Factors in Schizophrenia: Mechanisms and Experimental Approaches (2019) Mol Neuropsychiatry. 5:6-12. https://doi.org/10.1159/000495063
MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders (2007) Behav Brain Res 176: 149-69. https://doi.org/10.1016/j.bbr.2006.07.025
Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism (2008) Neuropharmacology 54: 901-911. https://doi.org/10.1016/j.neuropharm.2008.01.013
Shultz SR, Macfabe DF, Martin S, Jackson J, Taylor R, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impairs cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism (2009) Behav Brain Res 200: 33-34. https://doi.org/10.1016/j.bbr.2008.12.023
Hooks KB and OMalley MA. Dysbiosis and Its Discontents (2017) mBio 8: e01492-1517. https://doi.org/10.1128/mbio.01492-17
Davis IJ, Wallis C, Deusch O, Colyer A, Milella L, et al. A cross-sectional survey of bacterial species in plaque from client owned dogs with healthy gingiva, gingivitis or mild periodontitis (2013) PloS one 8: e83158. https://doi.org/10.1371/journal.pone.0083158
Yamasaki Y, Nomura R, Nakano K, Naka S, Matsumoto-Nakano M, et al. Distribution of periodontopathic bacterial species in dogs and their owners (2012) Arch Oral Biol 57: 1183-1188. https://doi.org/10.1016/j.archoralbio.2012.02.015
Hillman JD, McDonell E, Hillman CH, Zahradnik RT and Soni MG. Safety assessment of probiora3, a probiotic mouthwash: subchronic toxicity study in rats (2009) Int J Toxicol 28: 357-367. https://doi.org/10.1177/1091581809340705
Dye BA, Thornton-Evans G, Xianfen L and Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012 (2015) NCHS Data Brief 197.
Meurman JH and Stamatova I. Probiotics: contributions to oral health (2007) Oral Diseases 13: 443-451. https://doi.org/10.1111/j.1601-0825.2007.01386.x
Cildir SK, Germec D, Sandalli N, Ozdemir FI, Arun T, et al. Reduction of salivary mutans streptococci in orthodontic patients during daily consumption of yoghurt containing probiotic bacteria (2009) Eur J Orthod 31: 407-411. https://doi.org/10.1093/ejo/cjn108
Hedayati-Hajikand T, Lundberg U, Eldh C and Twetman. Effect of probiotic chewing tablets on early childhood caries-a randomized controlled trial (2015) BMC oral health 15: 112. https://doi.org/10.1186/s12903-015-0096-5
Ferreira AS, Silva-Paes-Leme AF, Raposo NR and da Silva SS.By passing microbial resistance: xylitol controls microorganisms growth by means of its anti-adherence property (2015) Curr Pharm Biotechnol 16: 35-42.
Ghezelbash GR, Nahvi I and Rabbani M. Comparative inhibitory effect of xylitol and erythritol on the growth and biofilm formation of oral Streptococci (2012) AJMR 6: 4404-4408.
Brambilla E, Ionescu AC, Cazzaniga G, Ottobelli M and Samaranayake LP. Levorotatory carbohydrates and xylitol subdue Streptococcus mutans and Candida albicans adhesion and biofilm formation (2015) J Basic Microbiol 56: 480-492. https://doi.org/10.1002/jobm.201500329
Sardi JC, Duque C, Mariano FS, Peixoto IT, Höfling JF, et al. Candida spp. in periodontal disease: a brief review (2010) J Oral Sci 52: 177-185.https://doi.org/10.2334/josnusd.52.177
De-La-Torre J, Quindós G, Marcos-Arias C, Marichalar-Mendia X, Gainza ML, et al. Oral candida colonization in patients with chronic periodontitis. Is there any relationship (2018) Rev Iberoam Micol 35: 134-139. https://doi.org/10.1016/j.riam.2018.03.005
Rodrigues CF, Rodrigues ME and Henriques M. Candida sp. infections in patients with diabetes mellitus (2019) J Clin Med 8: 76. https://doi.org/10.3390/jcm8010076
Vargas SL, Patrick CC, Ayers GD and Hughes WT. Modulating effect of dietary carbohydrate supplementation on Candida albicans colonization and invasion in a neutropenic mouse model (1993) Infect Immun 61: 619-626.
Van Ende M, Wijnants S and Van Dijck P. Sugar sensing and signaling in Candida albicans and Candida glabrata (2019) Front Microbiol 10: 99. https://doi.org/10.3389/fmicb.2019.00099
Bartnicka D, Karkowska-Kuleta J, Zawrotniak M, Satała D, Michalik K, et al. Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment (2019) Sci Rep 9: 4376. https://doi.org/10.1038/s41598-019-40771-8
Ichikawa T, Yano Y, Fujita Y, Kashiwabara T and Nagao K. The enhancement effect of three sugar alcohols on the fungicidal effect of benzethonium chloride toward Candida albicans (2008) J Dent 36: 965-968. https://doi.org/10.1016/j.jdent.2008.07.013
Kim J, Yoon-Young Kim, Ji-Youn Chang and Hong-Seop Kho. Candidacidal activity of xylitol and sorbitol (2016) J Oral Med Pain 41: 155-160 https://doi.org/10.14476/jomp.2016.41.4.155
Lim JH, Jeong Y, Song SH, Ahn JH, Lee JR, et al. Penetration of an antimicrobial zinc-sugar alcohol complex into Streptococcus mutans biofilms (2018) Scientific Reports 8: 16154. https://doi.org/10.1038/s41598-018-34366-y
Han SJ, Jeong SY, Nam YJ, Yang KH, Lim HS, et al. Xylitol inhibits inflammatory cytokine expression induced by lipopolysaccharide from Porphyromonas gingivalis (2005) Clin Diagn Lab Immunol 12: 1285-1291. https://doi.org/10.1128/CDLI.12.11.1285-1291.2005
Park E, Na HS, Kim SM, Wallet S, Cha S, et al. Xylitol, an anticaries agent, exhibits potent inhibition of inflammatory responses in human THP-1-derived macrophages infected with Porphyromonas gingivalis (2014) J Periodontol 85: e212-e223. https://doi.org/10.1902/jop.2014.130455
.Harjola U and Liesmaa H. Effects of polyol and sucrose candies on plaque, gingivitis and lactobacillus index scores (1978) Acta Odontologica Scandinavica 36: 237-242.
Tenovuo J, Mielityinen H and Paunio K.Effect of dental plaque grown in the presence of xylitol or sucrose on bone resorption in vitro (1981) Pharmacol Ther Dent 6: 35-43.
Mielityinen H, Tenovuo J, Söderling E and Paunio K. Effect of xylitol and sucrose plaque on release of lysosomal enzymes from bones and macrophages in vitro (1983) Acta Odontol Scand 41: 173-180.
Shyama M, Honkala E, Honkala S and Al-Mutawa SA.Effect of xylitol candies on plaque and gingival indices in physically disabled school pupils (2006) Clin Dent 17: 17-21.
Hashino E, Kuboniwa M, Alghamdi SA, Yamaguchi M, Yamamoto R, et al. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonas gingivalis (2013) Mol Oral Microbiol 28: 435-451. https://doi.org/10.1111/omi.12037
How KY, Song KP and Chan KG. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line (2016) Frontiers in microbiology 7: 53. https://doi.org/10.3389/fmicb.2016.00053
Zenobia C and Hajishengallis G. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis (2015) Virulence 6: 236-243. https://doi.org/10.1080/21505594.2014.999567
Boesten DM, Berger A, De Cock P, Dong H, Hammock BD, et al. Multi-targeted mechanisms underlying the endothelial protective effects of the diabetic-safe sweetener erythritol (2013) PLoS One 8: e65741. https://doi.org/10.1371/journal.pone.0065741
Nayak PA, Nayak UA and Khandelwal V. The effect of xylitol on dental caries and oral flora (2014) Clin Cosmet Investig Dent 6: 89-94. https://doi.org/10.2147/CCIDE.S55761
Falony G, Honkala S, Runnel R, Olak J, Nõmmela R, et al. Long-term effect of erythritol on dental caries development during childhood: a post-treatment survival analysis (2016) Caries Res 50: 579-588. https://doi.org/10.1159/000450762
Ur-Rehman S, Mushtaq Z, Zahoor T, Jamil A and Murtaza MA. Xylitol: a review on bioproduction, application, health benefits, and related safety issues (2015) Crit Rev Food Sci Nutr 55: 1514-1528. https://doi.org/10.1080/10408398.2012.702288
Cock PD. Erythritol functional roles in oral-systemic health (2018) Advances in Dental Research 29: 104-109. https://doi.org/10.1177/0022034517736499
Pussinen PJ, Paju S, Koponen J, Viikari JSA, Taittonen L, et al. Association of Childhood Oral Infections With Cardiovascular Risk Factors and Subclinical Atherosclerosis in Adulthood (2019) JAMA Netw Open 2: e192523. https://doi.org/10.1001/jamanetworkopen.2019.2523
Hillman JD, McDonell E, Hillman CH, Zahradnik RT, Soni MG (2009) Safety assessment of ProBiora3, a probiotic mouthwash; subchronic toxicity study in rats. Int J Toxicol 28(5): 357-367.
Walsh CJ, Guinane CM, OToole PW and Cotter PD. Beneficial modulation of the gut microbiota (2014) FEBS Lett 588: 4120-4130. https://doi.org/10.1016/j.febslet.2014.03.035
